This page provides a comprehensive overview of the fermentation value chain for alternative protein production. It includes details about the processes involved in precision fermentation and biomass fermentation and explains the workflow from R&D to commercialisation. Additionally, it discusses the various jobs required at each stage, the skills necessary for these roles, and where to acquire them. Whether you are a student, a professional looking to transition into the industry, or simply interested in learning more about the field, this page will help guide you in the right direction.




Fermentation for alternative protein production encompasses both biomass fermentation and precision fermentation. Biomass fermentation involves the cultivation of microorganisms such as fungi, algae, or bacteria to produce large quantities of protein-rich biomass. This biomass can then be processed into various food products. Precision fermentation, on the other hand, uses genetically engineered microorganisms to produce specific proteins or other biomolecules with high precision. These proteins can mimic those found in animal products or can be entirely novel, providing unique functional and nutritional benefits. Together, fermentation technologies offer a sustainable and scalable method for producing alternative proteins, which can complement or enhance existing plant-based and cultivated meat alternatives.
The fermentation-derived alternative protein field is at the forefront of food technology innovation, requiring a diverse array of skilled professionals to drive its progress. Talented individuals with expertise in microbiology, molecular biology, biochemistry, and chemical engineering are essential for optimising fermentation processes and engineering strains for higher yields and better performance. Additionally, food technologists, process engineers, and quality assurance experts are needed to scale up production and ensure the consistency and safety of the final products. Marketing professionals, regulatory specialists, and supply chain managers also play critical roles in bringing these novel products to market. The collaboration of multidisciplinary teams will be crucial to overcoming technical challenges and meeting consumer demand.
Working on fermentation-derived alternative protein production offers the opportunity to make a significant impact on both the environment and public health. Fermentation-derived proteins are more efficient and sustainable to produce than conventional animal proteins, reducing demand for land and water, and resulting in lower greenhouse gas emissions. Furthermore, fermentation technologies can address food security by providing a reliable and scalable source of high-quality protein. By developing and producing affordable and delicious alternative proteins, professionals in this field are driving a more sustainable and resilient food system and promoting better public health outcomes.


The technology value chain map details the step-by-step processes involved in production of alternative proteins through fermentation. Users can click on each step to learn more about the specific processes, equipment used, and jobs associated with that stage. The map is divided into key stages of development, making it easy to follow the workflow from strain development and upstream processing to downstream processing and product development.

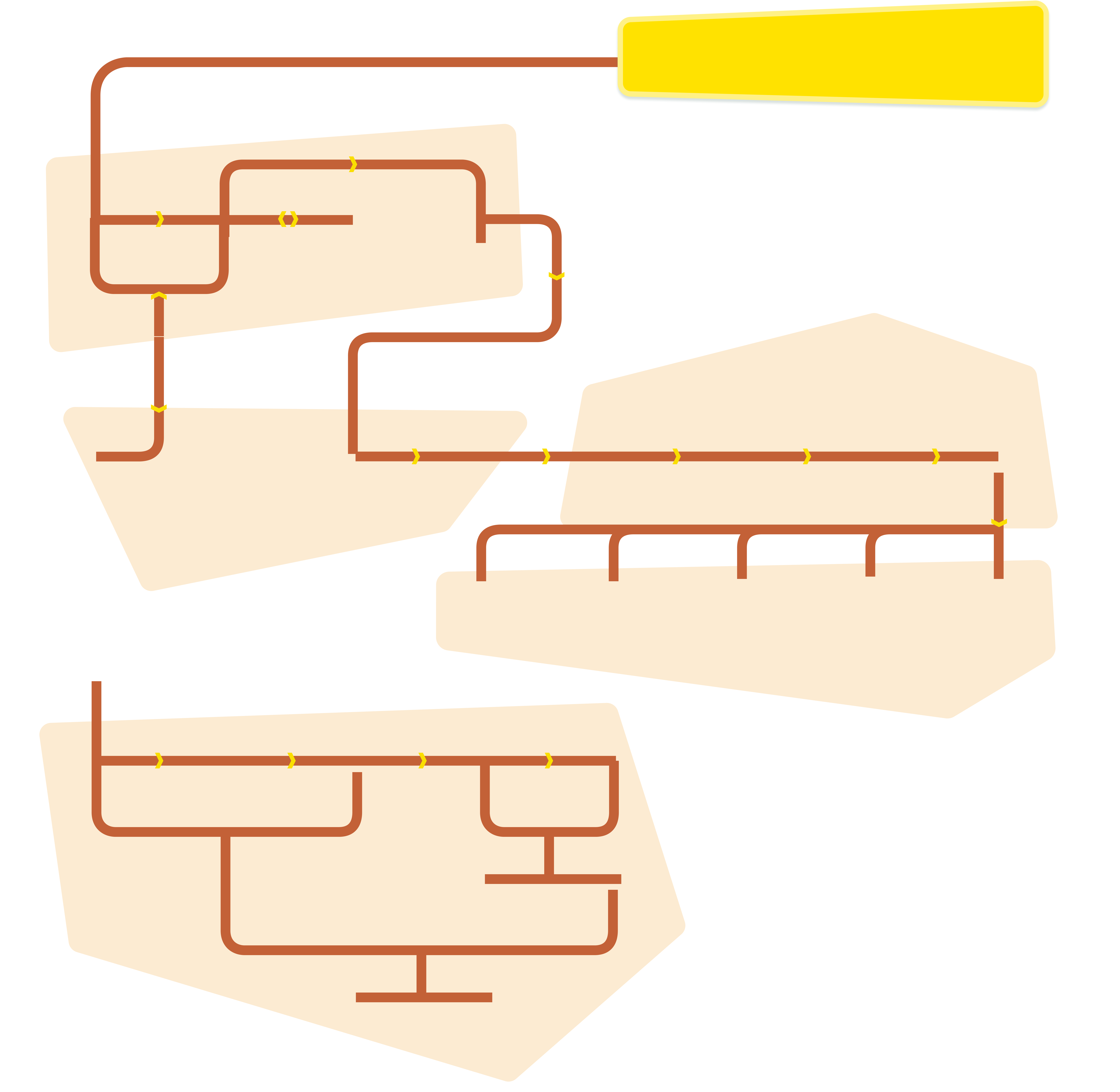
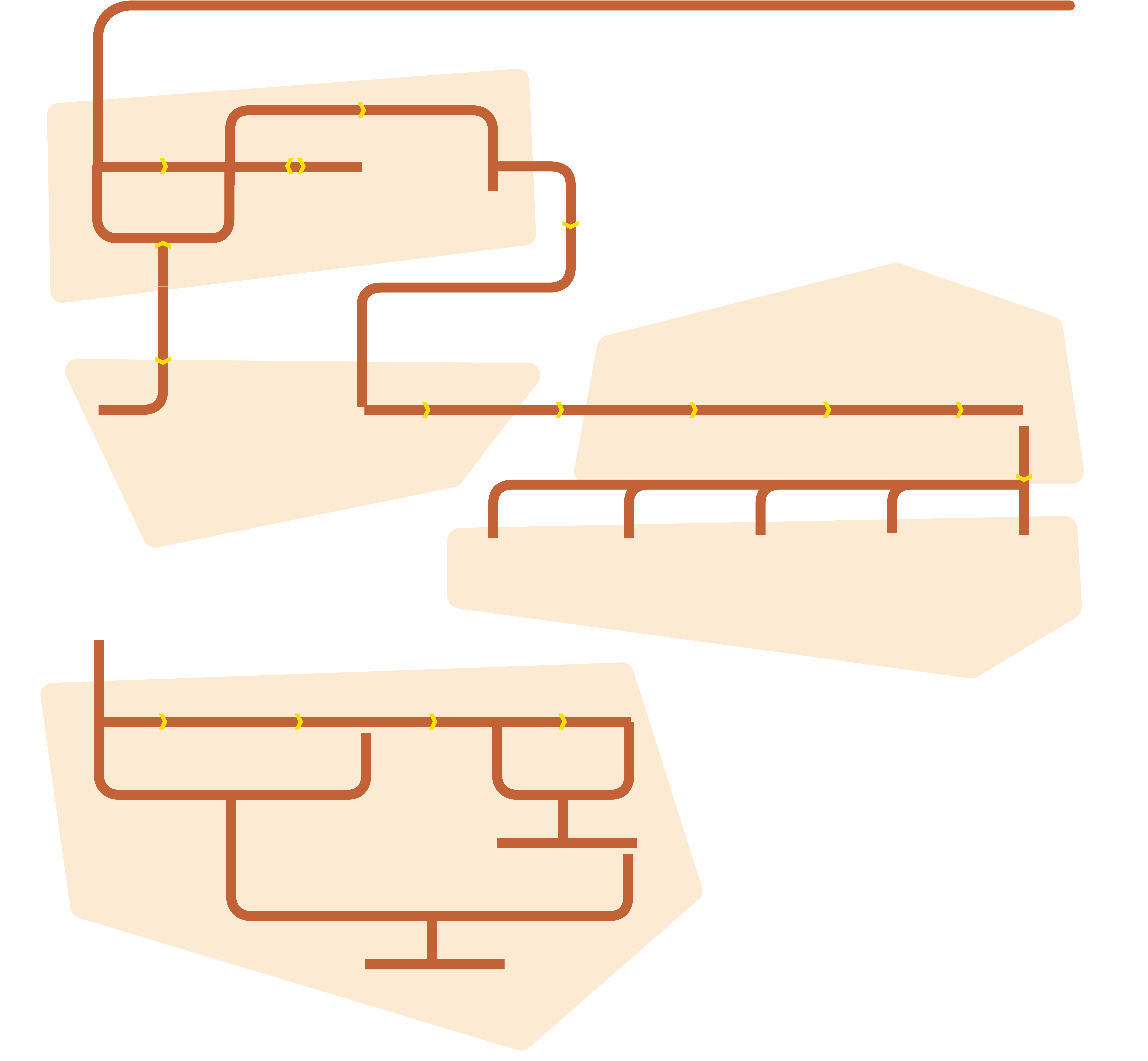






The technology value chain map details the step-by-step processes involved in the production of alternative proteins through fermentation. Users can click on each step to learn more about the specific activities, equipment used, and jobs associated with that stage. The map is divided into key stages of development, making it easy to follow the workflow from strain development and upstream processing to downstream processing and product development.
This section delves into the various job archetypes within the fermentation-derived alternative protein industry. It provides detailed descriptions of each role and outlines their responsibilities, the skills required, and the typical backgrounds that are a good fit. Additionally, it offers guidance on where to bridge any skill gaps, making it a valuable resource for those looking to enter or advance in the field.






Fermentation Scientists’ primary responsibility is to design, conduct, and analyse fermentation experiments to enhance the yield and quality of the desired protein product. They select and optimise microbial strains, develop fermentation media, and fine-tune process parameters such as temperature, pH, and agitation to maximise productivity.
On a daily basis, Fermentation Scientists collaborate with Strain Engineers to identify the best-performing microbial strains and with Bioprocess Engineers to scale up fermentation processes from lab-scale to pilot and commercial scales. They are involved in setting up and monitoring bioreactors, analysing fermentation kinetics, and troubleshooting any issues that arise during the process. Their work also includes maintaining detailed records of experiments and results, preparing technical reports, and presenting findings to the broader team.
The end goal for a Fermentation Scientist is to develop robust, scalable, and efficient fermentation processes that consistently produce high-quality alternative proteins. They need a strong background in microbiology, biochemistry, and chemical engineering, as well as practical experience with fermentation technology. Technical skills in bioreactor operation, process optimisation, and analytical techniques are crucial for their role.
Discover more global alternative protein courses and programmes through GFI’s comprehensive Alternative Protein Course Database.
Use this database to find up-to-date listings of available positions in the alternative protein ecosystem, including at GFI’s international affiliates. We also recommend exploring the Tälist and Alt Protein Careers job boards, which highlight a vast array of high-impact opportunities in the field.











Strain Engineers focus on the genetic modification and optimisation of microbial strains used in fermentation processes. Their primary responsibility is to develop strains that have enhanced characteristics such as higher protein yields, faster growth rates, or the ability to utilise alternative feedstocks. They use techniques like CRISPR, gene cloning, and metabolic engineering to introduce and optimise genetic traits.
In their daily operations, Strain Engineers collaborate with Fermentation Scientists to test the performance of engineered strains under various fermentation conditions. They design and execute experiments to evaluate the impact of genetic modifications and work on troubleshooting any issues related to strain stability or performance. Their projects often involve high-throughput screening of mutant libraries, metabolic pathway analysis, and bioinformatics to identify and optimise target genes.
The end goal for a Strain Engineer is to create robust, high-yielding microbial strains that are suitable for large-scale fermentation processes. They need a deep understanding of molecular biology, genetics, and metabolic engineering. Technical skills in genetic manipulation, metabolic pathway optimisation, and bioinformatics are essential for their role.
Discover more global alternative protein courses and programmes through GFI’s comprehensive Alternative Protein Course Database.
Use this database to find up-to-date listings of available positions in the alternative protein ecosystem, including at GFI’s international affiliates. We also recommend exploring the Tälist and Alt Protein Careers job boards, which highlight a vast array of high-impact opportunities in the field.














Purification Scientists are responsible for developing and optimising processes to isolate and purify proteins and other products from fermentation broths. Their primary task is to design and implement purification protocols that yield high-purity products while maximising recovery rates and maintaining cost-effectiveness. They work with various techniques such as chromatography, filtration, and centrifugation to achieve this goal.
In their daily operations, Purification Scientists collaborate with Fermentation Scientists to understand the characteristics of the fermentation broth and with Formulation Scientists to ensure the purified product meets the required specifications. They conduct experiments to optimise purification parameters, scale up purification processes, and troubleshoot any issues that arise. Their work involves maintaining detailed records of experiments, preparing technical reports, and presenting findings to the broader team.
The end goal for a Purification Scientist is to develop efficient and scalable purification processes that produce high-quality alternative protein products. They need a strong background in biochemistry, chemical engineering, and process engineering. Technical skills in chromatography, filtration, and analytical techniques are crucial for their role.
Discover more global alternative protein courses and programmes through GFI’s comprehensive Alternative Protein Course Database.
Use this database to find up-to-date listings of available positions in the alternative protein ecosystem, including at GFI’s international affiliates. We also recommend exploring the Tälist and Alt Protein Careers job boards, which highlight a vast array of high-impact opportunities in the field.













Bioprocess Engineers are integral to the development and optimisation of the fermentation processes used in the production of alternative proteins. They design and refine the systems and operations that convert raw materials into valuable products through biological processes. Their responsibilities include scaling up fermentation processes, optimising bioreactor conditions, and integrating automation to ensure efficiency and consistency.
On a daily basis, Bioprocess Engineers work closely with Fermentation Scientists to translate lab-scale processes to pilot and commercial scales. They are involved in setting up and monitoring bioreactors, optimising parameters such as temperature, pH, and agitation, and troubleshooting any operational issues. They also collaborate with Purification Scientists to ensure that downstream processes are seamlessly integrated with upstream operations.
The end goal for a Bioprocess Engineer is to develop robust, scalable, and cost-effective bioprocesses that consistently produce high-quality alternative protein products. They need a deep understanding of chemical engineering principles, bioprocess design, and process control. Technical skills in bioreactor operation, process optimisation, and data analysis are crucial for their role.
Discover more global alternative protein courses and programmes through GFI’s comprehensive Alternative Protein Course Database.
Use this database to find up-to-date listings of available positions in the alternative protein ecosystem, including at GFI’s international affiliates. We also recommend exploring the Tälist and Alt Protein Careers job boards, which highlight a vast array of high-impact opportunities in the field.













Quality assurance (QA) and Quality control (QC) professionals are essential in maintaining the safety, efficacy, and consistency of biomass and precision-fermented proteins. QA focuses on preventing defects through process improvements and adherence to quality standards, while QC involves rigorous testing of products to ensure they meet specified criteria. Their responsibilities include developing quality management systems, conducting audits, and performing routine product testing.
In their daily operations, QA/QC professionals design and implement quality management protocols, inspect and audit fermentation processes, and test samples of raw materials and finished products. They collaborate closely with production teams to maintain quality standards throughout the manufacturing process and with Regulatory Affairs staff to ensure compliance with relevant regulations. Their projects often involve developing standard operating procedures (SOPs), conducting root cause analysis of quality issues, and implementing corrective actions.
The end goal for QA/QC professionals is to ensure that all fermentation-derived protein products are safe, effective, and of high quality. They require strong analytical skills, attention to detail, and a thorough understanding of quality management principles. Technical skills in analytical chemistry, microbiology, and quality management systems are essential for their role.
Discover more global alternative protein courses and programmes through GFI’s comprehensive Alternative Protein Course Database.
Use this database to find up-to-date listings of available positions in the alternative protein ecosystem, including at GFI’s international affiliates. We also recommend exploring the Tälist and Alt Protein Careers job boards, which highlight a vast array of high-impact opportunities in the field.








As a subscriber, you’ll receive GFI APAC’s monthly newsletter as well as occasional updates and opportunities to support and get involved in our work. You may unsubscribe at any time. Please see our Privacy Policy for more details
As a subscriber, you’ll receive GFI APAC’s monthly newsletter as well as occasional updates and opportunities to support and get involved in our work. You may unsubscribe at any time. Please see our Privacy Policy for more details
Strain selection is the first step for both biomass and precision fermentation processes. In theory, microbial fermentation encompasses an enormous variety of species with vast biological diversity, ranging from fungi to bacteria to microalgae. However, very few microbial species have so far been commercialised for food use.
As biomass fermentation focuses on cultivating microbial biomass as a protein source, the selected strain must grow rapidly and produce high protein levels and essential nutrients. Common microbes used in biomass fermentation include fibrous fungi like Fusarium venenatum, other mycoprotein sources, and algae like Chlorella and Spirulina species. These microbes can vary in nutritional profile, growth and yield efficiency, final texture, and function.
Precision fermentation harnesses microorganisms (e.g., yeast, bacteria, fungi) to produce specific ingredients that can be used in various food and agricultural products. The ingredients produced can include egg and dairy proteins as well as specific enzymes, flavours, colours, fats, and oil. Strains are therefore selected for their ability to produce the target molecule efficiently and at scale. In the target selection process, the molecule or molecules of interest are called the target. The target can be a protein, a lipid, a flavour compound, a fragrance, an enzyme, a growth factor, a pigment, or another class of molecule. The chosen microbe, also referred to as the host organism, must efficiently grow these molecules in the upstream process and then be separated and purified from the target molecule in the downstream process. Scientists must carefully choose the ideal microbe for the entire precision fermentation process. Common microbes include fungi like Trichoderma reesei and Komagataella phaffii and bacteria like Escherichia coli.
Strains can be sourced from various culture collections and commercial suppliers. Comprehensive strain discovery programmes rely on massive data sets and specimen libraries, or biofoundries, to ensure broad capture of sufficient biological diversity, genomic data, and distinct growth conditions.
In both types of fermentation, the strain selection process is crucial for optimising yield, scalability, and the final product’s functionality. The chosen strains are often subjected to genetic modifications to enhance their performance and ensure that they meet the specific demands and safety requirements of alternative protein production.
High-throughput screening systems | Microplate readers | Microbial culture libraries |
Automated liquid handling systems | Incubators |
High-throughput screening systems |
Automated liquid handling systems |
Microbial culture libraries |
Microplate readers |
Incubators |
Strain engineering involves genetically modifying microbial strains to enhance their protein production capabilities, which is crucial for both biomass and precision fermentation. Advanced biotechnological tools like CRISPR-Cas9, homologous recombination, and transposon mutagenesis are employed to introduce specific genetic changes.
CRISPR-Cas9 allows for precise genetic modifications by creating targeted breaks in DNA. The process begins with identifying the gene or genetic sequence that influences traits like protein yield or metabolic efficiency. Guide RNA (gRNA) is designed to match the sequence adjacent to the gene of interest, directing the Cas9 enzyme to the exact location for editing. Once the Cas9 makes a cut, the cell’s natural DNA repair mechanisms are leveraged to introduce new genetic material, delete segments, or alter the existing sequence.
Identifying target genes involves analysing metabolic pathways using bioinformatics tools to determine which genes to knock out, insert, or modify for enhanced protein production. Scientists might target various features, such as:
After engineering, strains undergo extensive testing to ensure that the modifications enhance performance without compromising growth or stability. The goal is to create microbial strains that produce high yields of target proteins under industrial fermentation conditions, making them more efficient and reliable for alternative protein production. Successful strain engineering bridges the gap between basic research and practical application, which ensures that the strains meet the specific demands of industrial-scale production.
Bioreactors for small-scale cultures | PCR machines | CRISPR-Cas9 kits |
Sequencing equipment | Electroporators |
Sequencing equipment |
PCR machines |
Bioreactors for small-scale cultures |
Electroporators |
CRISPR-Cas9 kits |
Strain optimisation is a critical process that fine-tunes genetically engineered strains to maximise efficiency and stability in protein production. This stage utilises techniques like Adaptive Laboratory Evolution (ALE) and codon optimisation to ensure that strains function at their most efficient state.
In ALE, strains are exposed to selective pressures—such as temperature, pH, nutrient availability, and oxygen levels—over multiple generations to identify and cultivate those with optimal performance. High-throughput screening and automated systems evaluate environmental conditions and genetic modifications simultaneously.
Codon optimisation is particularly crucial in precision fermentation. It involves modifying the DNA sequence of the target gene to use codons preferred by the host organism, increasing protein expression, efficient protein folding, and post-translational modification without altering the protein’s amino acid sequence. Tailoring gene sequences to the microbial host’s preferred codons significantly enhances protein production efficiency.
Omics technologies—such as genomics, transcriptomics, proteomics, and metabolomics—help understand the strains’ metabolic pathways and regulatory networks, offering comprehensive insights for further refinement.
After optimisation, strains are validated in pilot-scale bioreactors to ensure that they maintain their enhanced traits under conditions mimicking large-scale production. This validation confirms the optimised strains’ ability to deliver consistent, high-yield production in commercially relevant conditions.
Automated fermentation systems | High-throughput screening platforms | Real-time polymerase chain reaction machines |
Chromatography systems | Incubators |
Automated fermentation systems |
High-throughput screening platforms |
Real-time polymerase chain reaction machines |
Chromatography systems |
Incubators |
Cell banking is a crucial process in fermentation, as it ensures the availability of consistent, high-quality microbial strains for production. This involves creating a Master Cell Bank (MCB) and Working Cell Banks (WCBs) from a well-characterised and optimised microbial strain. The MCB serves as the original source, stored under conditions that preserve genetic and phenotypic stability, while WCBs are derived from the MCB and used for routine production, thus maintaining consistency across batches.
The creation of cell banks begins with the extensive characterisation of the optimised strain, which ensures genetic stability, growth consistency, and freedom from contamination. The selected strain is expanded under controlled conditions, aliquoted, and cryopreserved using cryoprotectants such as glycerol or dimethylsulfoxide at ultra-low temperatures (e.g., -80°C or in liquid nitrogen at -196°C). This process ensures the long-term viability and retention of the desired traits for future use.
Role of Biofoundries: Biofoundries, which are comprehensive data and specimen libraries, play a vital role in modern cell banking. They enable high-throughput, automated processes for strain development and storage and ensure precision and scalability. Biofoundries are also essential for systematic strain discovery, which allows for the broad capture of biological diversity, genomic data, and growth conditions.
Challenges and Opportunities: Developing and maintaining comprehensive strain libraries and biofoundries is expensive and time-consuming, often restricting access to a few well-funded entities. These limitations can delay the commercial adoption of novel strains and hinder broader innovation. Coordinated efforts in biofoundries can accelerate the discovery and optimisation of strains suitable for alternative protein production by providing extensive data for strain improvement and adaptation, facilitating faster regulatory approval and commercial adoption of new strains. Such efforts can drive significant improvements in nutritional quality, production efficiency, strain robustness, and end-product traits.
To overcome these challenges, the industry must focus on both technological advancements and the adoption of globally recognised standards for feedstock and strain quality. Comprehensive efforts should also integrate systems biology insights to design novel hosts with ideal attributes for fermentation, such as prolonged stability, efficient metabolism, and desirable flavour profiles.
Cryopreservation systems | Liquid nitrogen storage tanks | Cell culture incubators |
Automated cell counters | Cryoprotectant solutions |
Sequencing equipment |
PCR machines |
Bioreactors for small-scale cultures |
Electroporators |
CRISPR-Cas9 kits |
Feedstock and media optimisation are critical in developing nutrient formulations that support the efficient growth and productivity of selected microbial strains in biomass and precision fermentation. The media typically consist of carbon and nitrogen sources, vitamins, minerals, and growth factors essential for microbial metabolism and protein synthesis. Depending on the microbial strain, the media’s composition is adjusted to match specific nutritional needs and optimise the yield of the target product.
In biomass fermentation, the feedstock must support economically efficient microbial growth, with a focus on high protein content. Refined sugar-based substrates from corn or sugarcane are commonly used due to their availability and well-characterised nature. The fermentation media often includes a blend of carbon sources such as glucose, sucrose, dextrose, and lactose for energy, along with nitrogen sources like ammonium salts, urea, amino acids, and yeast extract for protein synthesis. Essential minerals such as magnesium, calcium, phosphates, iron, and zinc play crucial roles in enzymatic functions and cellular processes. Additionally, vitamins and growth factors, including B vitamins, biotin, folic acid, and trace elements like selenium and copper, are necessary for optimal microbial metabolism and protein yield. As sustainability and cost-effectiveness gain importance, alternative feedstocks—such as agricultural byproducts, waste streams, and gaseous substrates—are being explored. For example, fungi used in biomass fermentation can be grown on lignocellulosic feedstocks, which are abundant and economical, though more challenging to process.
In precision fermentation, where microbes are engineered to produce specific molecules like proteins or enzymes, the media is tailored to enhance the expression and stability of the target molecule. Carbon sources like glucose, methanol, and glycerol, along with precisely formulated nitrogen sources such as ammonium salts and amino acids, are used to support the expression of target proteins. Minerals are carefully balanced, with iron, magnesium, and trace elements being critical for enzyme co-factors and metabolic pathways. Growth factors and vitamins are customised, often including specialised cofactors essential for biosynthesis. The media is optimised for the specific microbial host to maximise yield, efficiency, and product purity. For instance, in producing dairy proteins using yeast, the media might be optimised to increase the secretion of casein or whey protein while maintaining yeast cell health and productivity.
The optimisation process involves empirical testing and computational modelling. Researchers test various feedstocks and media components to identify those that maximise yield and minimise production costs. Computational tools model microbial metabolic pathways, predicting how different media compositions will affect growth and production.
The ultimate goal is to create a scalable, cost-effective, and sustainable fermentation process, ensuring that microbial strains can be grown at an industrial scale without compromising efficiency or product quality.
Bioreactors | Analytical blances | HPLC systems |
Spectrophotometers | Media preparation vessels |
Bioreactors |
Analytical blances |
HPLC systems |
Spectrophotometers |
Media preparation vessels |
Media preparation is a crucial step in the upstream process, involving the formulation and sterilisation of growth media used in fermentation. This step ensures that the media is free from contaminants and contains all the necessary nutrients for optimal microbial growth. Large-scale preparation equipment, such as media tanks and autoclaves, are used to mix and sterilise the media. The media composition is carefully optimised based on the results from the feedstock and media optimisation step. Proper pH adjustment and nutrient balance are critical to support the high-density cultures required for protein production. The prepared media is then transferred to sterile storage tanks or directly into the bioreactors for fermentation. This step ensures that the microbial cultures have a consistent and nutrient-rich environment that promotes efficient protein production.
Media recycling is increasingly explored as a strategy to reduce costs and waste, particularly in large-scale fermentation processes. However, recycling presents challenges, such as maintaining nutrient balance and preventing the accumulation of inhibitory byproducts that could hinder microbial growth. Effective recycling requires careful monitoring and adjustment of the media composition to ensure that it continues to support optimal microbial activity.
Supply chain optimisation is another critical aspect, as the availability and cost of raw materials can significantly impact the overall economics of the fermentation process. Sourcing sustainable and affordable feedstocks, such as agricultural byproducts or waste streams, is a priority to enhance the sustainability of fermentation operations. This approach not only reduces reliance on refined sugars but also promotes a circular economy by utilising waste materials as valuable inputs. Ensuring a stable and cost-effective supply of these materials is essential for scaling up fermentation processes and achieving long-term viability.
Media preparation tanks | Sterile filtration units | Autoclaves |
PH meters | Mixing vessels |
Media preparation tanks |
Sterile filtration units |
Autoclaves |
PH meters |
Mixing vessels |
Cleaning and sterilisation are vital processes in maintaining the safety and efficiency of fermentation operations. Proper sterilisation ensures that all microbial life, including potential contaminants, is eradicated from equipment and surfaces before the initiation of a new fermentation batch. Autoclaving is a common sterilisation method that uses high-pressure steam to achieve this, ensuring that bioreactors, tubing, and other equipment are free from any biological contaminants that could compromise the fermentation process.
In the context of fermentation involving living modified organisms (LMOs), strict regulatory compliance is required to prevent biohazards. The Cartagena Protocol on Biosafety provides international guidelines for the safe handling, transport, and use of LMOs, which ensures that organisms do not pose risks to human health or the environment. Compliance with these regulations is crucial for companies involved in the development and production of alternative proteins, as it ensures that their operations are safe and legally compliant.
Safety protocols are paramount in cleaning and sterilisation efforts, not only to protect the integrity of fermentation processes, but also to safeguard workers and the environment. Proper handling of biohazardous materials, including the use of personal protective equipment, biosafety cabinets, and containment strategies, is essential to prevent accidental exposure or release of harmful organisms. Regular validation of sterilisation procedures, such as through biological indicators and sterility testing, is also necessary to confirm that sterilisation processes are effective and meet industry standards.
In addition to autoclaving, other sterilisation methods such as steam-in-place (SIP) systems, chemical sterilants, and UV irradiation may be employed depending on the specific requirements of the process and the properties of the materials being sterilised. Maintaining rigorous standards for cleaning and sterilisation not only supports regulatory compliance but also ensures the smooth operation of fermentation processes, minimising the risk of contamination and production downtime.
Validation sensors | Steam Generators | Clean-in-place (CIP) and Steam-in-place (SIP) systems |
Chemical sterilants | Autoclaves |
Validation sensors |
Steam Generators |
Chemical sterilants |
Autoclaves |
Clean-in-place (CIP) and Steam-in-place (SIP) systems |
Monitoring and control are critical components in ensuring the success of fermentation processes. This involves the continuous observation and regulation of key parameters such as temperature, pH, dissolved oxygen, and nutrient levels within the bioreactor. These parameters must be maintained within strict ranges to optimise microbial growth and product formation, as deviations can lead to reduced yields or compromised product quality.
Advanced sensors and monitoring systems are integrated into the bioreactor setup to provide real-time data on these critical parameters. For instance, temperature sensors ensure that the fermentation environment remains within the optimal range for the specific microbial strain, while pH sensors monitor the acidity or alkalinity of the broth, which can significantly impact enzyme activity and metabolic processes. Dissolved oxygen sensors are particularly important in aerobic fermentation processes, as they measure the availability of oxygen, which is essential for the respiration and growth of aerobic microbes.
Nutrient levels are also closely monitored to ensure that the microbial culture is adequately supplied with carbon, nitrogen, and other essential nutrients throughout the fermentation process. Automated nutrient dosing systems can be employed to adjust the feed rates based on real-time data, thereby ensuring that the microbes have a consistent supply of nutrients without the risk of overfeeding, which could lead to byproduct formation or inhibition of microbial growth.
Effective monitoring and control systems not only maximise the efficiency and yield of the fermentation process but also contribute to the overall quality and consistency of the final product. By collecting and analysing data throughout the fermentation process, operators can make informed decisions and adjustments to optimise the process. This continuous feedback loop is essential for maintaining the stability of the fermentation environment, reducing variability, and ensuring that the product meets the desired specifications.
Bioreactor control systems | Data acquisition software | PH and oxygen probes |
Automated control units | In-line sensors |
Bioreactor control systems |
Data acquisition software |
PH and oxygen probes |
Automated control units |
In-line sensors |
Seed train development is a critical step in scaling up microbial cultures for fermentation, beginning with a small volume of starter culture and progressively expanding it to larger vessels. The process starts with inoculating small flasks, which are then scaled up through a series of small-scale bioreactors. The seed train process has many variations and can involve progressively scaling from 1, 3, 5, 10 to 20 litres to 1, 10, 100, 1000, 10k, and 100k litres. The purpose of the seed train is to increase both the density and the volume of the microbial culture, which ensures that the microbes are actively replicating and producing the target molecule, whether that be a protein, enzyme, or another desired compound.
As the seed train progresses, the microbial cells adapt to increasing volumes and more complex environmental conditions. Throughout this process, cells undergo rapid growth and proliferation, particularly during the log phase, where the population expands exponentially. This expansion is supported by carefully controlled conditions, including nutrient-rich fermentation broth, which serves as the medium in which the microbes grow. The fermentation broth will be a complex mixture of microbes, water, carbon sources, nitrogen sources, vitamins, minerals, and growth factors that provide the necessary nutrients for microbial metabolism and biosynthesis.
By the end of the seed train process, the culture is prepared for transfer into the primary bioreactor, where large-scale production will occur. This step is vital for ensuring that the culture is robust and dense enough to handle the transition to large-scale bioreactors without losing its productivity or stability.
Shake flasks | Seed bioreactors | Incubators |
Cell counters | Media preparation vessels |
Shake flasks |
Seed bioreactors |
Incubators |
Cell counters |
Media preparation vessels |
Primary USP bioreactor cultivation is where microbial cultures are scaled up to industrial volumes, often ranging from hundreds to thousands of litres, to maximise the production of proteins, enzymes, or biomass. This stage is critical in transitioning from seed train development to full-scale fermentation, where the focus is on maintaining optimal conditions such as temperature, pH, dissolved oxygen, and nutrient supply to ensure efficient microbial growth and target molecule production. The bioreactor setup, microbe cultivation process, and media formulation will differ depending on the fermentation technique used. Types of fermentation include:
Aerobic vs. Anaerobic Fermentation: The choice between aerobic and anaerobic fermentation significantly impacts the process. Aerobic fermentation, which requires oxygen, generally yields higher biomass or target molecules and produces byproducts like carbon dioxide. However, it is energy-intensive due to the need for continuous oxygen supply and cooling systems to manage the heat generated. Anaerobic fermentation occurs without oxygen and produces ethanol, lactic acid, or other metabolites as byproducts. This method is typically slower and yields less biomass but is more energy-efficient and often used in specific precision fermentation applications. Each method’s choice depends on the desired product and the microbial strain used, with aerobic processes commonly applied in high-value products like proteins and anaerobic processes in applications like bioethanol production.
Temperature Control and Cooling: Maintaining the correct temperature is vital for microbial growth and product formation. Overheating generated during fermentation can negatively impact yield and process efficiency. Cooling systems are integrated into bioreactors to dissipate heat and maintain the desired temperature. This is crucial as even slight deviations from the optimal temperature can slow microbial activity or lead to the degradation of target molecules. Different fermentation setups may require unique cooling solutions depending on the type of fermentation and the specific process conditions.
Fermentation Times and Yields: The duration of upstream fermentation varies based on the process and organism used. SmF processes typically last from 24 to 72 hours, while SSF and LAIF can extend over several days or weeks. Yield, often measured as grams of product per litre of culture (g/L), is a critical metric for process efficiency. Higher yields reduce production costs and improve scalability, making yield optimisation a key focus in bioprocess development.
Large-scale bioreactors | In-line sensors | Automated feeding systems |
Temperature control units | Aeration systems |
Large-scale bioreactors |
In-line sensors |
Automated feeding systems |
Temperature control units |
Aeration systems |
Harvesting is the first step in downstream processing (DSP). DSP involves purifying and refining the target product from the fermentation broth, which may include proteins, enzymes, or biomass, depending on the type of fermentation used. Harvesting specifically refers to the initial step where the microbial culture is collected from the bioreactor, setting the stage for subsequent purification and processing. DSP is essential for transforming the raw fermentation broth with the target molecule into a final, market-ready product with high purity and quality.
Harvesting Based on Fermentation Type:
Efficient harvesting is crucial as it determines the yield and quality of the final product. The choice of harvesting method impacts the purity of the product and the efficiency of the subsequent downstream processes. Properly conducted harvesting minimises the loss of valuable target molecules and reduces contamination risks, ensuring that the downstream processing can proceed smoothly and efficiently.
Centrifuges | Filtration units | Sedimentation tanks |
Harvesting pumps | Storage containers |
Centrifuges |
Filtration units |
Sedimentation tanks |
Harvesting pumps |
Storage containers |
Extraction is first phase of separation, which removes the target product—such as proteins, enzymes, or other molecules—from the harvested microbial biomass or fermentation broth, as well as cellular components, impurities, and residual media, thereby setting the foundation for further purification and refinement.
Different types of extraction techniques can be used as follows:
Efficient extraction is crucial for maximising protein yield and purity. Proper extraction methods ensure that the maximum amount of the target product is recovered from the fermentation broth or biomass while minimising contaminants. This step also preserves the activity and stability of the product, which is essential for its functionality in end-use applications. The extraction process must balance efficiency, cost, and product quality.
Homogenisers | Lysis buffers | Enzymatic digestion kits |
Filtration units | Extraction tanks |
Homogenisers |
Lysis buffers |
Enzymatic digestion kits |
Filtration units |
Extraction tanks |
Separation and purification followed by extraction, aims to isolate and purify the target product—such as proteins, enzymes, or other molecules—at high specificity and purity levels. These processes ensure that the final product meets the stringent quality requirements necessary for food applications or other end uses.
The level of purity required depends on the intended application of the product. For example, food-grade proteins need to be free from contaminants like other proteins, nucleic acids, lipids, and residual solvents. High specificity is essential to ensure that the target molecule is effectively separated from other components in the mixture.
Different types of purification techniques can be employed including:
Purification steps can be costly, especially with techniques like chromatography that require specialised resins and equipment. The choice of method must balance cost with the required purity and specificity. High costs can be mitigated by optimising the process, such as by reusing chromatography resins, or by developing more cost-effective alternatives.
The efficiency of the separation and purification steps directly affects the overall yield of the process. High yields are critical for ensuring the economic viability of the production process, particularly in large-scale operations.
Chromatography systems | Ultrafiltration units | Precipitation tanks |
Analytical instruments | Purification columns |
Chromatography systems |
Ultrafiltration units |
Precipitation tanks |
Analytical instruments |
Purification columns |
The final step in DSP is drying, preservation, and storage, where the purified product is converted into a stable form suitable for long-term storage and transport. This step is crucial for maintaining the quality and functionality of the target molecule or biomass, ensuring it remains viable for its intended application in food products or other uses. Different techniques include:
Once the target ingredient has been dried, the following preservation techniques can be used to ensure long-term shelf life.
Finally, storing the product at appropriate temperature, moisture, and choosing the right packaging are key for creating the final product.
Efficient drying and preservation processes maximise product recovery and ensure that the target proteins or biomass retain their desired functional properties.
Drying and preservation are energy-intensive processes, with costs depending on the method used. Balancing cost with the need for high-quality, stable products is a key challenge. Freeze drying, while producing high-quality results, is costly, whereas spray drying offers a more cost-effective solution but may compromise some product quality.
Spray dryers | Freeze dryers | Lyophilisation units |
Storage containers | Moisture analysers |
Spray dryers |
Freeze dryers |
Lyophilisation units |
Storage containers |
Moisture analysers |
Ingredient characterisation involves analysing the physicochemical properties of the purified proteins to ensure that they meet the required specifications for food applications. This step includes evaluating factors such as protein content, molecular weight, solubility, thermal stability, product purity, and functional properties. Advanced analytical techniques such as mass spectrometry, high-performance liquid chromatography (HPLC), and differential scanning calorimetry (DSC) are used to characterise the target molecules.
Mass Spectrometry (MS): MS is used to determine the molecular weight and composition of proteins. It ionises the protein molecules and measures their mass-to-charge ratio, allowing for precise identification and characterisation of the protein structure, including post-translational modifications. This technique is critical for verifying the protein’s identity and ensuring it matches the desired specifications.
High-Performance Liquid Chromatography (HPLC): HPLC separates, identifies, and quantifies individual components in a mixture, making it vital for assessing product purity. In ingredient characterisation, HPLC is often used to detect impurities, quantify protein concentration, and assess the quality of the final product. It provides high-resolution separation, ensuring that the target protein is pure and free from contaminants.
Differential Scanning Calorimetry (DSC): DSC measures the thermal stability of proteins by assessing their heat capacity as they are heated. This technique provides critical information on the protein’s denaturation temperature and thermal transitions, which are important for understanding how the protein will behave during food processing and storage. It helps ensure that the protein will maintain its structure and functionality under various temperature conditions.
Solubility Testing: Solubility testing evaluates how well the protein dissolves in different solvents, which is crucial for determining its applicability in various food formulations. Proteins that are not sufficiently soluble may not be suitable for certain food products, so this testing helps in optimising the use of the protein in diverse food applications.
Functional Property Analysis: This involves testing the protein for functional characteristics such as emulsification, foaming, water-holding capacity, and gelation. These properties determine how the protein will perform in food products, affecting texture, stability, and overall quality. For example, a protein with good emulsifying properties is essential in products like mayonnaise or dressings.
Product Purity Testing: Ensuring product purity involves detecting and quantifying potential contaminants, such as other proteins, nucleic acids, or lipids. Techniques like sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) are often used alongside HPLC to verify that the protein meets the purity standards necessary for food use.
Proper characterisation methods are critical for producing high-quality proteins that meet the desired specifications for food applications. The characterised ingredients are then prepared for formulation and stability testing in subsequent steps.
Mass spectrometers | DSC instruments | Spectrophotometers |
HPLC systems | Protein analysers |
Mass spectrometers |
HPLC systems |
DSC instruments |
Protein analysers |
Spectrophotometers |
Formulation and stability testing are critical steps in developing stable, functional food products using characterised proteins. The process begins with optimising ingredient combinations, where the specific interactions between proteins, fats, carbohydrates, and other components are fine-tuned to achieve the desired product characteristics such as taste, texture, and nutritional value. Stability testing involves subjecting the formulated products to various environmental conditions, including fluctuations in temperature, humidity, and light, to assess how these factors affect the product’s shelf life and overall quality. This testing is essential for ensuring that the product maintains its intended sensory attributes and nutritional value over time, even under different storage and transportation conditions. The stability of the formulation is critical for determining the product’s market viability, as it influences the shelf life and consumer acceptance.
Emulsifiers | Stability chambers | Encapsulation units |
Texturisers | Analytical instruments |
Emulsifiers |
Texturisers |
Stability chambers |
Analytical instruments |
Encapsulation units |

Product labelling involves designing and creating labels that accurately represent the product’s ingredients, nutritional content, potential allergenicity, and other relevant information. This step ensures compliance with regulatory requirements and provides consumers with the information they need to make informed choices. Labelling also includes branding and marketing elements that communicate the product’s benefits and differentiators.
Nutritional analysis tools | Packaging materials | Design software |
Regulatory databases | Label printers |
Nutritional analysis tools |
Regulatory databases |
Packaging materials |
Label printers |
Design software |

Food safety and regulatory compliance are crucial for ensuring that alternative protein products meet the highest standards of safety and legality before reaching the market. This process involves rigorous safety assessments, certification processes, and regulatory submissions that vary depending on the country and region. For novel products—such as those developed through precision or biomass fermentation—regulatory approval can be especially challenging, requiring extensive testing to demonstrate safety and efficacy. This includes toxicology studies, allergenicity testing, and assessments of the nutritional profile.
Regulatory approval timelines can differ significantly across countries. For instance, the United States Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have distinct protocols and requirements for novel food ingredients. In some cases, products may need to undergo Generally Recognised as Safe (GRAS) determination in the U.S., or gain novel food approval in the EU. These processes involve comprehensive dossiers that document the safety, composition, and intended use of the product, and can take several years to complete.
Food safety is ensured through compliance with guidelines that identifies and mitigates potential hazards in the production process and final products. Specialised equipment is used to monitor critical parameters, thus ensuring that products are free from contaminants and meet quality standards. Regulatory submissions require detailed documentation of the manufacturing process, ingredient sourcing, and safety testing results.
Achieving food safety and regulatory compliance is essential not only for protecting consumers but also for securing the product’s marketability. Products that successfully navigate these rigorous processes are then prepared for the next stages, including quality assurance and post-processing.
Microbiological testing equipment | Certification documents | Compliance databases |
Regulatory submission software | Safety assessment tools |
Regulatory submission software |
Certification documents |
Microbiological testing equipment |
Safety assessment tools |
Compliance databases |

Quality assurance (QA) involves implementing systems and processes to ensure that the product consistently meets quality standards. QA activities include developing and documenting standard operating procedures (SOPs), conducting audits, and monitoring production processes. The goal is to prevent defects and ensure that the product is safe, effective, and of high quality. QA is an ongoing process that involves continuous improvement and adherence to best practices. Proper QA methods are critical for producing high-quality and market-ready food products.
QA software | SOP documentation tools | Monitoring sensors |
Audit tools | Statistical analysis software |
QA software |
SOP documentation tools |
Monitoring sensors |
Audit tools |
Statistical analysis software |
Post-processing texturising and flavouring involve enhancing the sensory attributes of the product to meet consumer preferences. Techniques such as extrusion, blending, and flavour encapsulation are used to create desirable textures and flavours. This step ensures that the final product is appealing to consumers in terms of taste, texture, and overall sensory experience. Advanced techniques like emulsification are employed to create stable mixtures of oil and water phases, which are essential for products like dressings and sauces. Encapsulation methods are used to protect sensitive ingredients like flavours, vitamins, or probiotics, ensuring their release at the appropriate time during consumption. Texturisation techniques help create the desired mouthfeel and structural integrity, such as mimicking the textures of conventional animal-based products. Post-processing also includes adding functional ingredients that improve the product’s nutritional profile. Proper post-processing texturising and flavouring methods are critical for producing high-quality, market-ready food products.
Sensory evaluation tools | Blenders | Flavour encapsulation equipment |
Pilot-scale processing equipment | Extruders |
Sensory evaluation tools |
Blenders |
Flavour encapsulation equipment |
Pilot-scale processing equipment |
Extruders |

Consumer testing and sensory analysis are crucial steps in the product development cycle for alternative protein products. The primary goals are to assess how well the product aligns with consumer preferences, identify areas for improvement in flavour, texture, aroma, appearance, and overall appeal, and gather insights into potential market reception.
Sensory attributes typically evaluated include:
Sample Sizes and Testing Methodology: Testing usually involves a mix of trained sensory panels and a larger group of untrained consumers representing the target audience. The size of the consumer panel can range from 50 to several hundred participants, depending on the scope of the test. Trained panels often include 10-20 experts, while larger consumer tests might involve 100-200 participants for more comprehensive feedback.
Participants are typically asked to rate each attribute on a scale (e.g., 1-10) or provide qualitative feedback. Common methods include:
Data from these tests are analysed both statistically and qualitatively. Statistical analysis helps identify trends, correlations, and significant differences between product variants. Qualitative insights provide context to the numerical data, offering deeper understanding of consumer preferences.
Based on the results, the product formulation may be adjusted, followed by further rounds of testing. This iterative approach ensures that the final product is fine-tuned to meet consumer expectations, increasing its chances of market success. By systematically gathering and analysing feedback, manufacturers can make informed decisions that enhance the product’s appeal and ensure it resonates with the intended market segment.
Sensory evaluation booths | Data collection software | Aroma analysis tools |
Tasting kits | Texture analysers |
Sensory evaluation booths |
Data collection software |
Aroma analysis tools |
Tasting kits |
Texture analysers |

At the commercial scale, establishing and maintaining a high-quality cell bank is crucial for ensuring consistent, reproducible fermentation processes. This involves creating and managing a Master Cell Bank (MCB) and Working Cell Bank (WCB) from optimised microbial strains. The MCB is the original source for production strains, stored under conditions that ensure genetic stability and long-term viability. WCBs are derived from the MCB and used for routine production, ensuring consistency across fermentation batches. Biofoundries play a significant role in maintaining these banks by providing extensive data and libraries for strain development. Regular assessments of viability and genetic stability are essential to ensure the ongoing quality of the cell bank, which serves as the foundation for large-scale production and regulatory compliance.
Liquid nitrogen storage tanks | Cryopreservation systems | Cryoprotectant solutions |
Cell culture incubators | Automated cell counters |
Liquid nitrogen storage tanks |
Cryopreservation systems |
Cryoprotectant solutions |
Cell culture incubators |
Automated cell counters |
At the commercial manufacturing scale, upstream processing (USP) involves scaling microbial cultures to volumes ranging from 10,000 to over 100,000 litres. This process can be conducted using batch or continuous processing modes. Batch processing involves inoculating the bioreactor, allowing the fermentation to proceed, and then harvesting the entire contents at the end of the cycle. It’s simpler and easier to control but may lead to downtime between batches, reducing overall productivity. On the other hand, continuous processing involves constantly feeding fresh media into the bioreactor while simultaneously harvesting the product, thereby maintaining cells in their most productive growth phase. This method can offer higher productivity and efficiency but requires more sophisticated monitoring and control systems.
Various bioreactors are used depending on the fermentation type, including stirred-tank bioreactors for submerged fermentation (SmF), bubble column and air-lift bioreactors for aerobic fermentation, and packed-bed and tray bioreactors for solid-state fermentation (SSF), which offer high surface area for microbial growth on solid substrates. Photobioreactors are used for processes involving light-dependent organisms. Gas or loop bioreactors are employed for specific gas-liquid interaction needs. The choice of bioreactor and processing mode significantly impacts the efficiency, scalability, and cost-effectiveness of the USP.
Large-scale bioreactors | Bioreactor control systems | In-line sensors |
Automated feeding systems | Data acquisition software |
Large-scale bioreactors |
Bioreactor control systems |
In-line sensors |
Automated feeding systems |
Data acquisition software |
Downstream processing (DSP) at the commercial manufacturing scale involves large-scale harvesting, extraction, separation, and purification of target proteins or biomass from bioreactors that can reach up to 100,000 litres in capacity. The DSP can also be conducted in batch or continuous processing modes. In batch DSP, all the product is harvested and processed in a single operation, making it easier to manage quality control but potentially causing bottlenecks and inefficiencies. Continuous DSP, on the other hand, allows for constant product extraction and purification, aligning with continuous USP, thus improving overall throughput and reducing downtime.
Harvesting techniques vary by bioreactor type and include centrifugation, filtration, or settling for liquid cultures, and mechanical separation for solid substrates in solid-state fermentation (SSF). Extraction methods like mechanical disruption (e.g., bead milling and sonication) or chemical lysis are used to release intracellular products. Separation and purification processes may involve chromatography (e.g., affinity and ion exchange), ultrafiltration, and precipitation to achieve the necessary purity and yield. The complexity and choice of methods are dictated by the type of fermentation, the specific product requirements, and the scale of production. Continuous DSP can improve efficiency and reduce costs, especially in large-scale operations, by ensuring a constant flow of product through the purification stages.
Large-scale centrifuges | Ultrafiltration units | Purification columns |
Chromatography systems | Precipitation tanks |
Large-scale centrifuges |
Ultrafiltration units |
Purification columns |
Chromatography systems |
Precipitation tanks |
In commercial-scale manufacturing, formulation and packaging processes must cater to both biomass and precision fermentation products, ensuring stability, functionality, and market readiness.
Formulation involves optimising ingredient combinations and processing methods for desired textures, flavours, and nutritional profiles. For biomass fermentation, the focus is on enhancing the taste and textural characteristics of the microbial biomass, while in precision fermentation, the emphasis is on achieving the target functionality of the produced ingredient. Techniques like emulsification, encapsulation, and texturisation are employed to create these final products that appeal to consumers.
Packaging plays a crucial role in preserving product quality during distribution. It must protect against environmental factors such as light, oxygen, and moisture, which could compromise the product’s stability and shelf life. Packaging materials are selected based on the product’s needs, with considerations for sustainability, cost, and consumer convenience. The choice of packaging impacts the product’s marketability and consumer acceptance.
The processes are closely monitored and adjusted to ensure that the final products retain their intended quality throughout their shelf life, making them ready for distribution and sale.
Stability chambers | Encapsulation units | Emulsifiers |
Analytical instruments | Packaging machines |
Stability chambers |
Encapsulation units |
Emulsifiers |
Analytical instruments |
Packaging machines |

Distribution at the commercial scale involves the logistics of transporting formulated and packaged products to markets and retail locations. This step ensures that products are delivered efficiently and in optimal condition, with specialised logistics solutions such as refrigerated transport and inventory management systems. Proper storage conditions and maintaining the cold chain during transit (when applicable) are crucial for preserving product quality and extending shelf life. Efficient distribution strategies are essential to meet market demand, minimise waste, and maximise profitability.
Inventory management systems | Refrigerated trucks | Logistics planning tools |
Distribution software | Storage containers |
Inventory management systems |
Refrigerated trucks |
Logistics planning tools |
Distribution software |
Storage containers |

Quality assurance (QA) involves implementing systems and processes to ensure that the product consistently meets quality standards. QA activities include developing and documenting standard operating procedures (SOPs), conducting audits, and monitoring production processes. The goal is to prevent defects and ensure that the product is safe, effective, and of high quality. QA is an ongoing process that involves continuous improvement and adherence to best practices. Proper QA methods are critical for producing high-quality and market-ready food products. This step involves the use of specialised equipment to ensure effective and efficient QA. The assured products are then prepared for Good Manufacturing Practices (GMP)/quality compliance in subsequent steps.
SOP documentation tools | QA software | Monitoring sensors |
Statistical analysis software | Audit tools |
SOP documentation tools |
QA software |
Monitoring sensors |
Statistical analysis software |
Audit tools |
Quality control (QC) at the commercial scale involves implementing robust systems to ensure products consistently meet quality standards. This includes inspecting, testing, and sampling products throughout production to detect and correct defects. QC is an ongoing process involving continuous monitoring, data collection, and adherence to best practices. Advanced analytical instruments and software are used to ensure that products meet the required specifications for safety, purity, and performance. QC at this scale is essential for maintaining consumer trust and regulatory compliance.
QC software | Sampling tools | Statistical analysis software |
Testing instruments | Inspection equipment |
QC software |
Sampling tools |
Statistical analysis software |
Testing instruments |
Inspection equipment |
Product labelling involves designing and creating labels that accurately represent the product’s ingredients, nutritional content, and other relevant information. This step ensures compliance with regulatory requirements and provides consumers with the information they need to make informed choices. Labelling also includes branding and marketing elements that communicate the product’s benefits and differentiators.
Nutritional analysis tools | Design software | Label printers |
Regulatory databases | Packaging materials |
Nutritional analysis tools |
Design software |
Label printers |
Regulatory databases |
Packaging materials |

Good Manufacturing Practices (GMP) and quality compliance involve adhering to regulatory standards and guidelines to ensure the safety and quality of the products. This step includes implementing and maintaining GMP systems, conducting regular audits, and ensuring compliance with regulatory requirements. GMP guidelines cover every aspect of the production process, from the sourcing of raw materials to the final product’s packaging and distribution. Key elements include:
GMP is the foundation of quality assurance in food production, ensuring that every product meets the required safety and quality standards before it reaches the consumer.
GMP documentation tools | Compliance software | Regulatory submission software |
Quality monitoring sensors | Audit tools |
GMP documentation tools |
Compliance software |
Regulatory submission software |
Quality monitoring sensors |
Audit tools |
Food safety and regulatory compliance are crucial for ensuring that alternative protein products meet the highest standards of safety and legality before reaching the market. This process involves rigorous safety assessments, certification processes, and regulatory submissions that vary depending on the country and region. For novel products—such as those developed through precision or biomass fermentation—regulatory approval can be especially challenging, requiring extensive testing to demonstrate safety and efficacy. This includes toxicology studies, allergenicity testing, and assessments of the nutritional profile.
Regulatory approval timelines can differ significantly across countries. For instance, the United States Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have distinct protocols and requirements for novel food ingredients. In some cases, products may need to undergo a Generally Recognised As Safe (GRAS) determination in the U.S., or gain novel food approval in the EU. These processes involve comprehensive dossiers that document the safety, composition, and intended use of the product, and can take several years to complete.
Food safety is ensured through compliance with guidelines that identify and mitigate potential hazards in the production process and final products. Specialised equipment is used to monitor critical parameters, thus ensuring that products are free from contaminants and meet quality standards. Regulatory submissions require detailed documentation of the manufacturing process, ingredient sourcing, and safety testing results.
Achieving food safety and regulatory compliance is essential not only for protecting consumers but also for securing the product’s marketability. Products that successfully navigate these rigorous processes are then prepared for the next stages, including quality assurance and post-processing.
Regulatory submission software | Regulatory guidance documents | Safety assessment tools |
Certification documents | Compliance databases |
Regulatory submission software |
Regulatory guidance documents |
Safety assessment tools |
Certification documents |
Compliance databases |


Strain Engineers focus on the genetic modification and optimisation of microbial strains used in fermentation processes. Their primary responsibility is to develop strains that have enhanced characteristics such as higher protein yields, faster growth rates, or the ability to utilise alternative feedstocks. They use techniques like CRISPR, gene cloning, and metabolic engineering to introduce and optimise genetic traits.
In their daily operations, Strain Engineers collaborate with Fermentation Scientists to test the performance of engineered strains under various fermentation conditions. They design and execute experiments to evaluate the impact of genetic modifications and work on troubleshooting any issues related to strain stability or performance. Their projects often involve high-throughput screening of mutant libraries, metabolic pathway analysis, and bioinformatics to identify and optimise target genes.
The end goal for a Strain Engineer is to create robust, high-yielding microbial strains that are suitable for large-scale fermentation processes. They need a deep understanding of molecular biology, genetics, and metabolic engineering. Technical skills in genetic manipulation, metabolic pathway optimisation, and bioinformatics are essential for their role.

Fermentation Scientists’ primary responsibility is to design, conduct, and analyse fermentation experiments to enhance the yield and quality of the desired protein product. They select and optimise microbial strains, develop fermentation media, and fine-tune process parameters such as temperature, pH, and agitation to maximise productivity.
On a daily basis, Fermentation Scientists collaborate with Strain Engineers to identify the best-performing microbial strains and with Bioprocess Engineers to scale up fermentation processes from lab-scale to pilot and commercial scales. They are involved in setting up and monitoring bioreactors, analysing fermentation kinetics, and troubleshooting any issues that arise during the process. Their work also includes maintaining detailed records of experiments and results, preparing technical reports, and presenting findings to the broader team.
The end goal for a Fermentation Scientist is to develop robust, scalable, and efficient fermentation processes that consistently produce high-quality alternative proteins. They need a strong background in microbiology, biochemistry, and chemical engineering, as well as practical experience with fermentation technology. Technical skills in bioreactor operation, process optimisation, and analytical techniques are crucial for their role.

Bioprocess Engineers are integral to the development and optimisation of the fermentation processes used in the production of alternative proteins. They design and refine the systems and operations that convert raw materials into valuable products through biological processes. Their responsibilities include scaling up fermentation processes, optimising bioreactor conditions, and integrating automation to ensure efficiency and consistency.
On a daily basis, Bioprocess Engineers work closely with Fermentation Scientists to translate lab-scale processes to pilot and commercial scales. They are involved in setting up and monitoring bioreactors, optimising parameters such as temperature, pH, and agitation, and troubleshooting any operational issues. They also collaborate with Purification Scientists to ensure that downstream processes are seamlessly integrated with upstream operations.
The end goal for a Bioprocess Engineer is to develop robust, scalable, and cost-effective bioprocesses that consistently produce high-quality alternative protein products. They need a deep understanding of chemical engineering principles, bioprocess design, and process control. Technical skills in bioreactor operation, process optimisation, and data analysis are crucial for their role.

Purification Scientists are responsible for developing and optimising processes to isolate and purify proteins and other products from fermentation broths. Their primary task is to design and implement purification protocols that yield high-purity products while maximising recovery rates and maintaining cost-effectiveness. They work with various techniques such as chromatography, filtration, and centrifugation to achieve this goal.
In their daily operations, Purification Scientists collaborate with Fermentation Scientists to understand the characteristics of the fermentation broth and with Formulation Scientists to ensure the purified product meets the required specifications. They conduct experiments to optimise purification parameters, scale up purification processes, and troubleshoot any issues that arise. Their work involves maintaining detailed records of experiments, preparing technical reports, and presenting findings to the broader team.
The end goal for a Purification Scientist is to develop efficient and scalable purification processes that produce high-quality alternative protein products. They need a strong background in biochemistry, chemical engineering, and process engineering. Technical skills in chromatography, filtration, and analytical techniques are crucial for their role.

Quality assurance (QA) and Quality control (QC) professionals are essential in maintaining the safety, efficacy, and consistency of biomass and precision-fermented proteins. QA focuses on preventing defects through process improvements and adherence to quality standards, while QC involves rigorous testing of products to ensure they meet specified criteria. Their responsibilities include developing quality management systems, conducting audits, and performing routine product testing.
In their daily operations, QA/QC professionals design and implement quality management protocols, inspect and audit fermentation processes, and test samples of raw materials and finished products. They collaborate closely with production teams to maintain quality standards throughout the manufacturing process and with Regulatory Affairs staff to ensure compliance with relevant regulations. Their projects often involve developing standard operating procedures (SOPs), conducting root cause analysis of quality issues, and implementing corrective actions.
The end goal for QA/QC professionals is to ensure that all fermentation-derived protein products are safe, effective, and of high quality. They require strong analytical skills, attention to detail, and a thorough understanding of quality management principles. Technical skills in analytical chemistry, microbiology, and quality management systems are essential for their role.